Plastic Engineering for Devices
Engineering-Driven Manufacturing for Medical Devices
We provide end-to-end plastic engineering for medical devices, from concept development to ISO 13485-certified production. Our integrated tooling, injection moulding, and validation workflows ensure quality, compliance, and scalability.

Design to Manufacturing Integration
We combine DFM/DFA principles, digital simulation, and precision tooling to streamline your product’s transition from concept to validated, manufacturable solution.

Certified Engineering Teams
With our certified specialists, your medical devices are in expert hands. We ensure they meet the stringent industry regulations, building trust and delivering positive impact.

Compliance Assurance
We embed IQ/OQ/PQ protocols, risk mitigation, and digital traceability into every production step to meet the strictest medtech regulatory and audit demands.
Manufacturing Partner for Medical Devices
We manufacture precision-moulded plastic components and housings for complex medical devices, optimised for dimensional accuracy, cleanroom performance, and regulatory compliance.

Drug Delivery
Pens, pumps, inhalers, auto-injectors

Surgical & Therapeutic
Specialised solutions in high-performance materials (PEEK, PSU, and implant-grade polymers).

Connected & Wearable
Sensor housings, smart device casings, wearable modules

Critical Care & Monitoring
Defibrillators, Blood test analyzers, Radiology meters, vital signs monitors
Integrated Plastic Solutions
Minimise Risk, Optimise
Design, and Accelerate
Time-to-Market
Count on seamless development of medical devices and solutions that improve lives.
Our certified experts empower your project with cost-efficiency, manufacturing
excellence, and faster time-to-market. You get compliance, reliability, and adherence
to the highest industry standards.
Portfolio & Case Studies
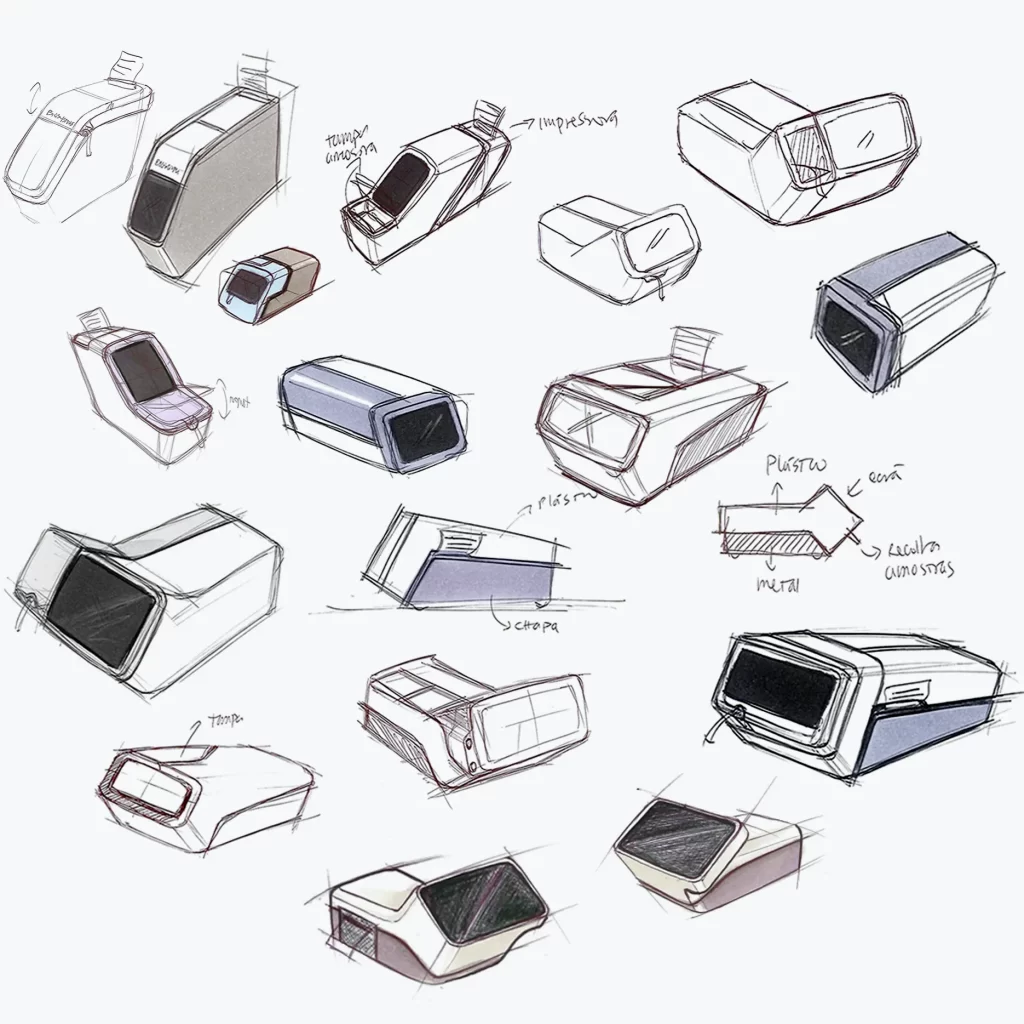
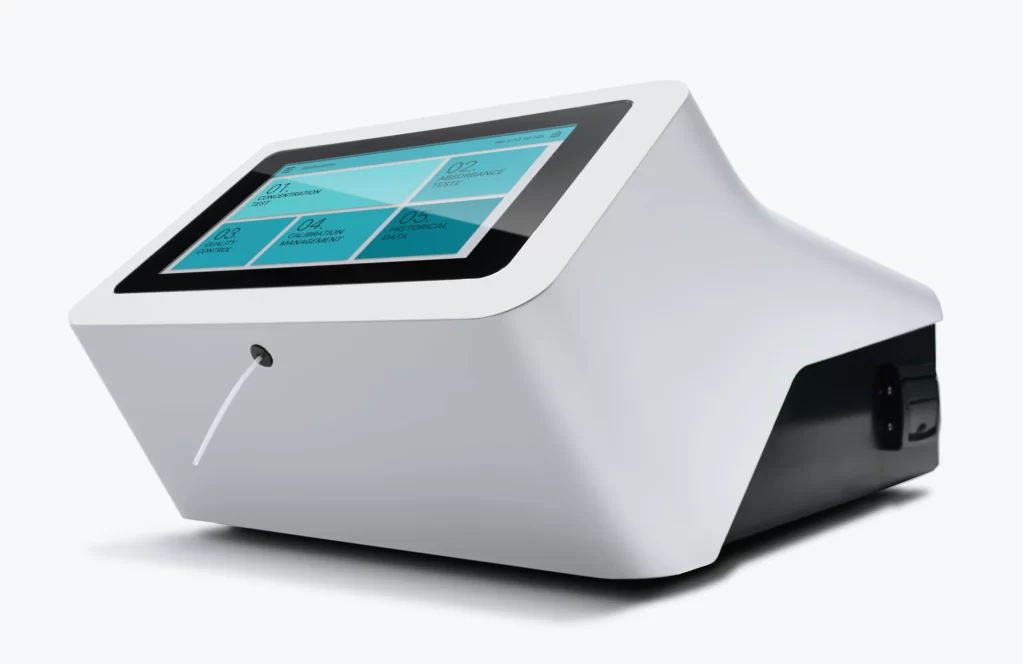
Semi-Automatic Analyzer BTS
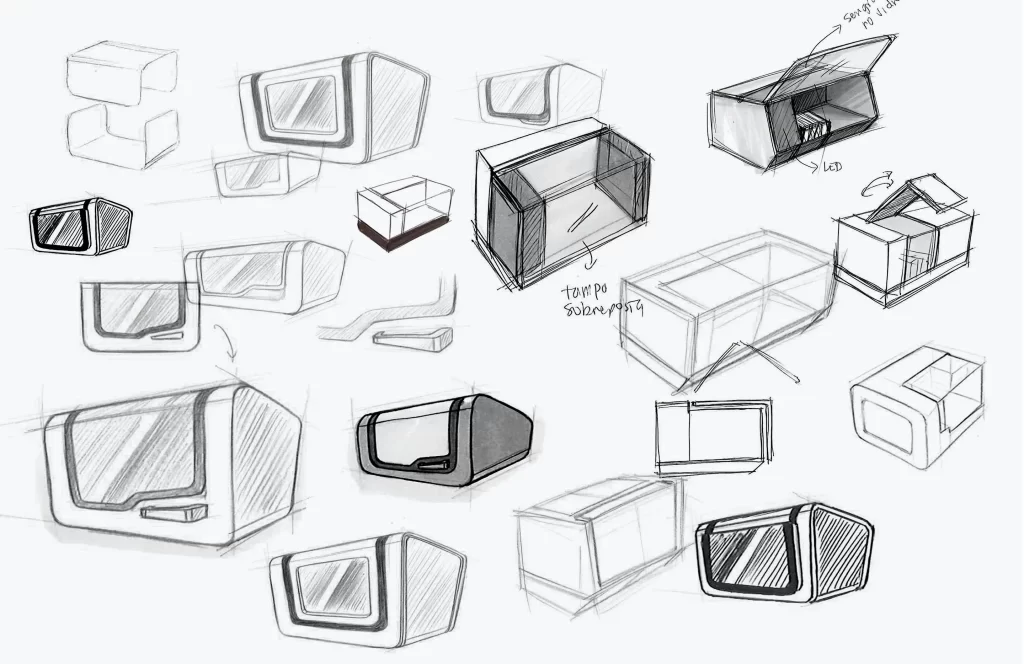
New Product Development, Engineering & Prototyping | User Interface
Client: Biosystems
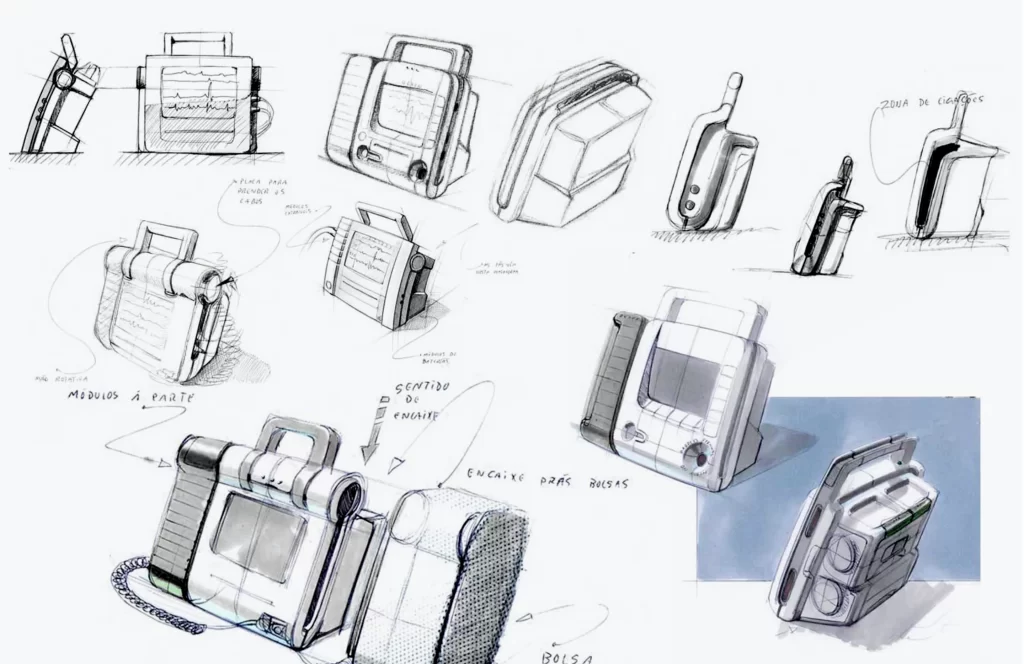
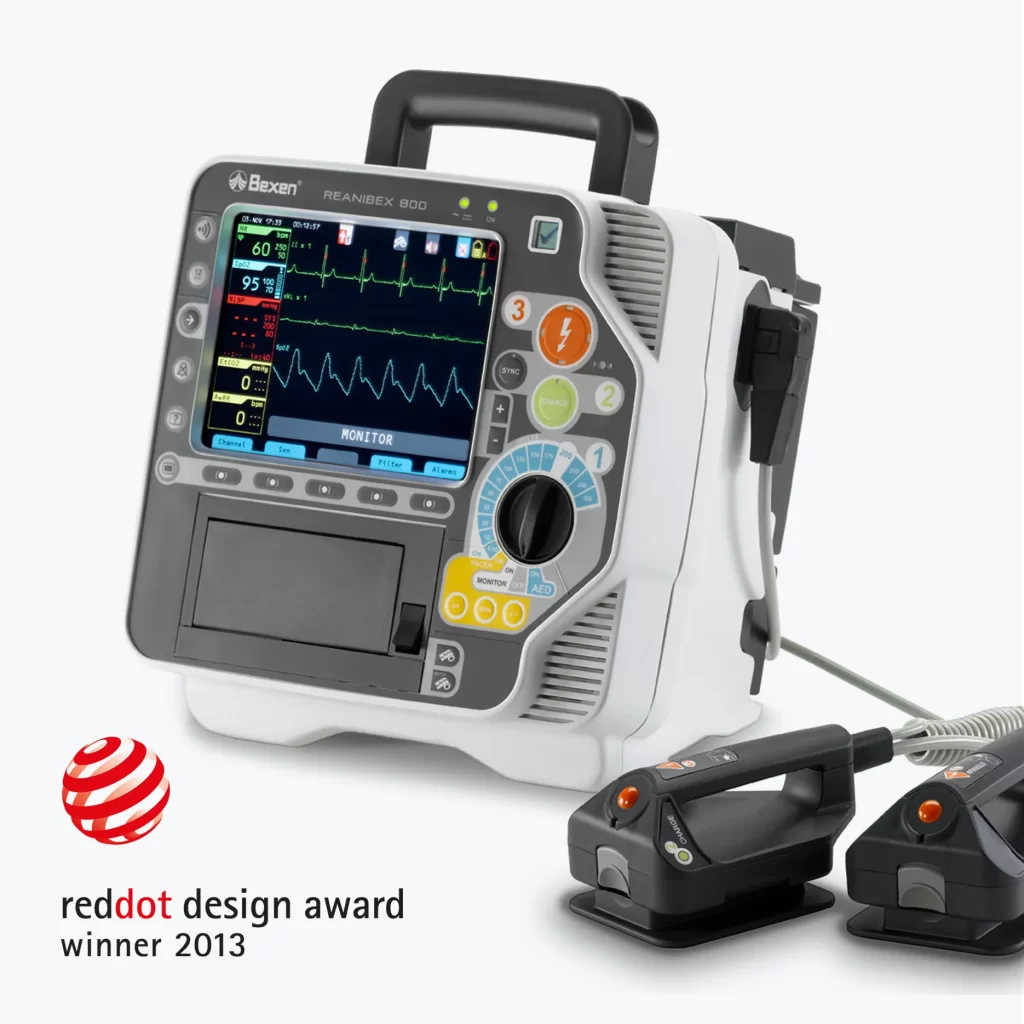
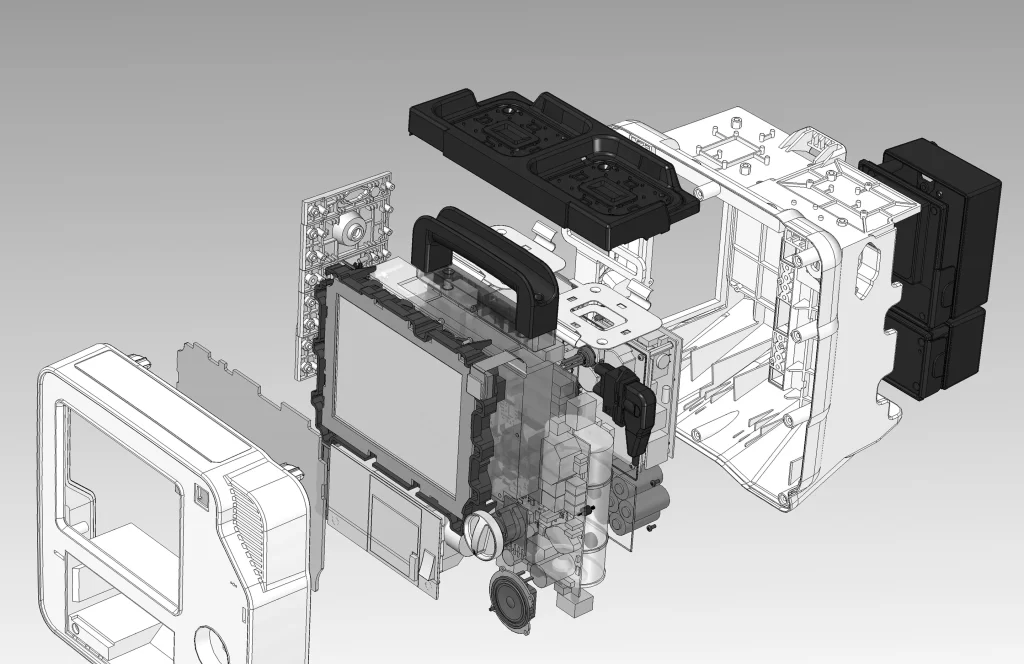
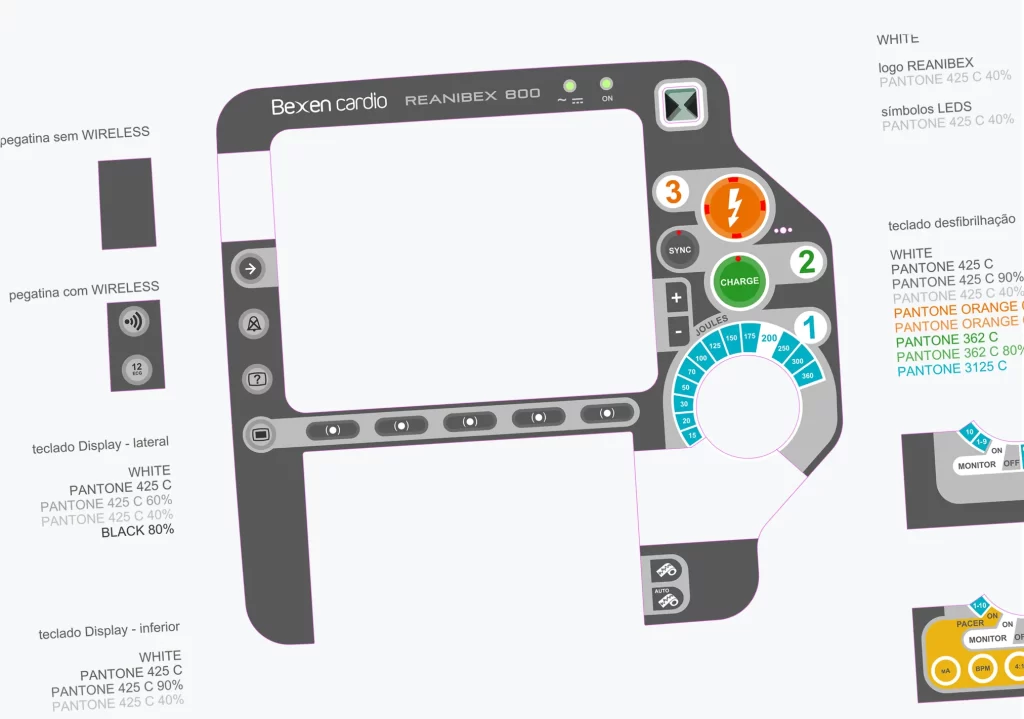
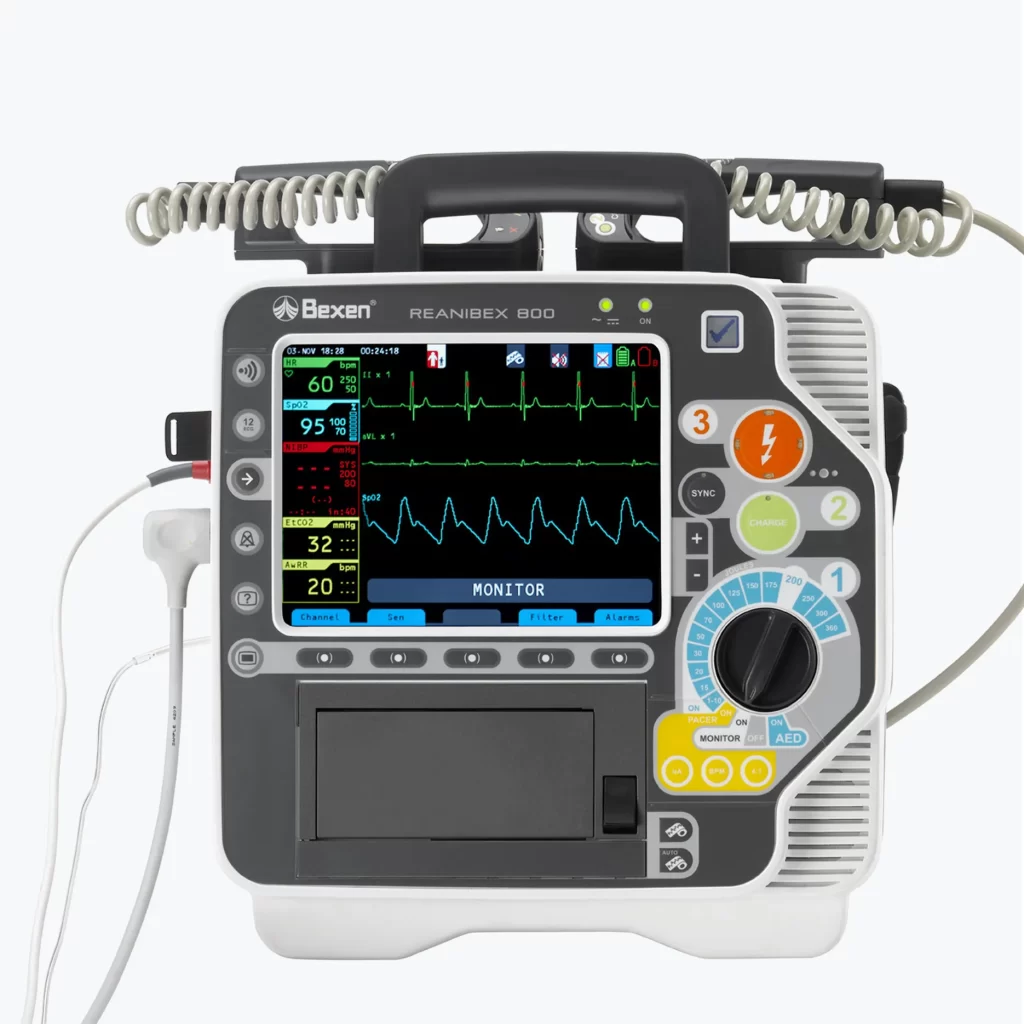
Monitor Defibrillator REANIBEX 800
New Product Development, Engineering & Prototyping | User Interface
Client: Bexencardio
Immunofluorescence Processor iPRO
New Product Development & Engineering
Client: Biosystems
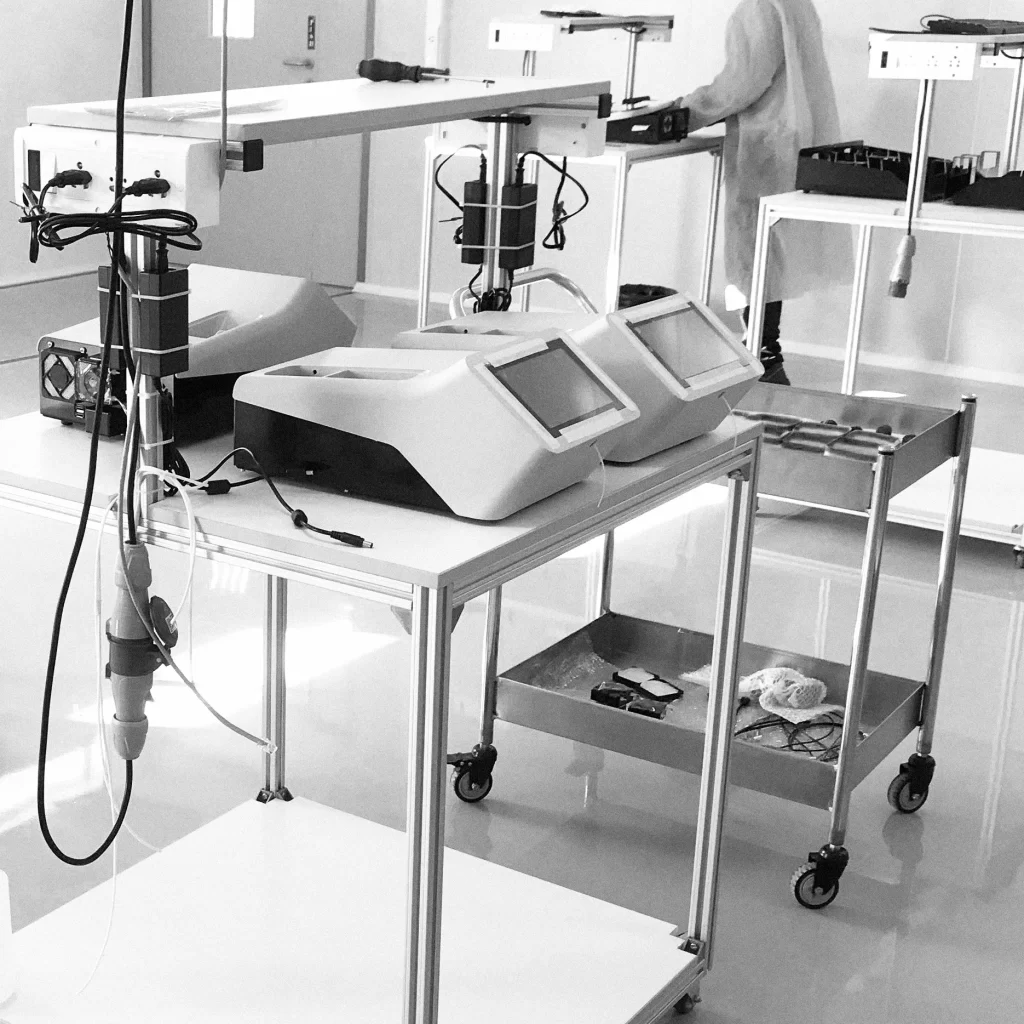
End-to-End Manufacturing Partner
for Certified Medical Products
We deliver fully integrated engineering, mould making, and ISO 13485-certified injection moulding, with robust validation and cleanroom capabilities tailored to the demands of regulated medical markets.
Full Product Lifecycle Integration
From concept to validated production, we streamline every step to reduce risk and accelerate readiness.
Regulatory-Compliant and Validated Manufacturing
Our manufacturing model is built on strict process validation (DQ, IQ, OQ, PQ), risk mitigation (DOE, FMEA), and cleanroom ISO 7 production, ensuring that every product meets global regulatory requirements and quality assurance standards for medical devices.
Advanced Mould Making and Injection Expertise
With decades of precision tooling and injection moulding experience, we deliver complex, high-tolerance components ready for scalable medical production. Our in-house capabilities ensure robust tool performance, dimensional stability, and repeatability in high-volume output.
Balanced Costs and Value through Design Optimisation
We apply DFM, DTC, DFA, and DTV principles to optimise manufacturability and cost without compromising quality.
Engineering with Simulation-Driven Decision Making
CAD, FEA, CFD, and Moldflow tools drive early-stage decisions that reduce iterations and improve long-term yield.