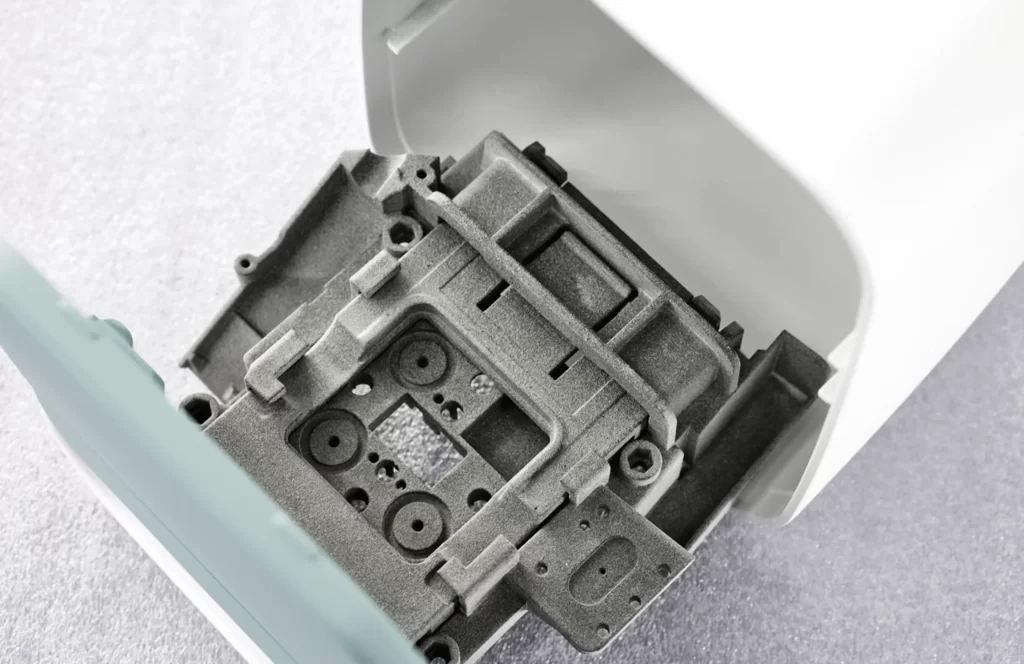
Plastic Engineering for Diagnostics
Precision Manufacturing for Diagnostics
From concept to value, we deliver injection-ready parts engineered for compliance, performance, and scalability throughout the lifecycle.

High-Precision Manufacturing
Accelerate time to market with advanced mould-making, validated injection, and ISO 13485-certified workflows designed for quality, scalability, and efficiency.

Tooling and Design Integration
From DFM to simulation, our engineering workflows ensure dimensional precision, repeatability, and optimized manufacturability from prototype to production.

Compliance Assurance
We embed compliance early, integrating process validation (IQ, OQ, PQ), traceability, and risk mitigation into every phase of development to meet the toughest global standards.
Manufacturing Partner for Diagnostic Excellence
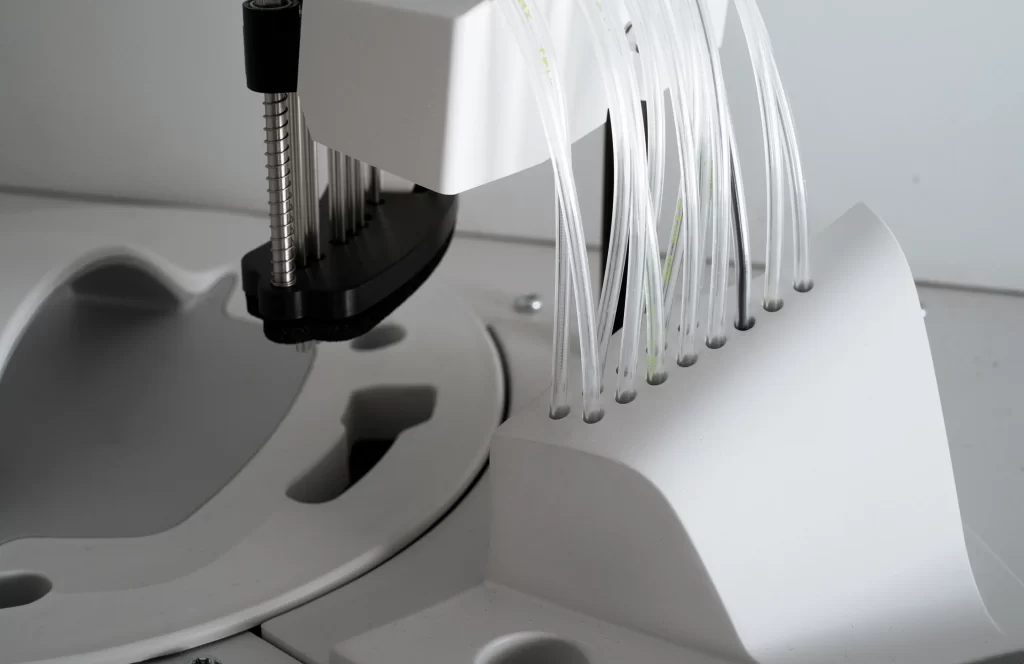
We support diagnostic innovation with precision-moulded plastic components, engineered for cleanliness, scalability, and compliance from prototyping to production.

Point-of-care Testing
Cartridges, cassette shells, and sample collection units, engineered for rapid diagnostics and clinical handling.

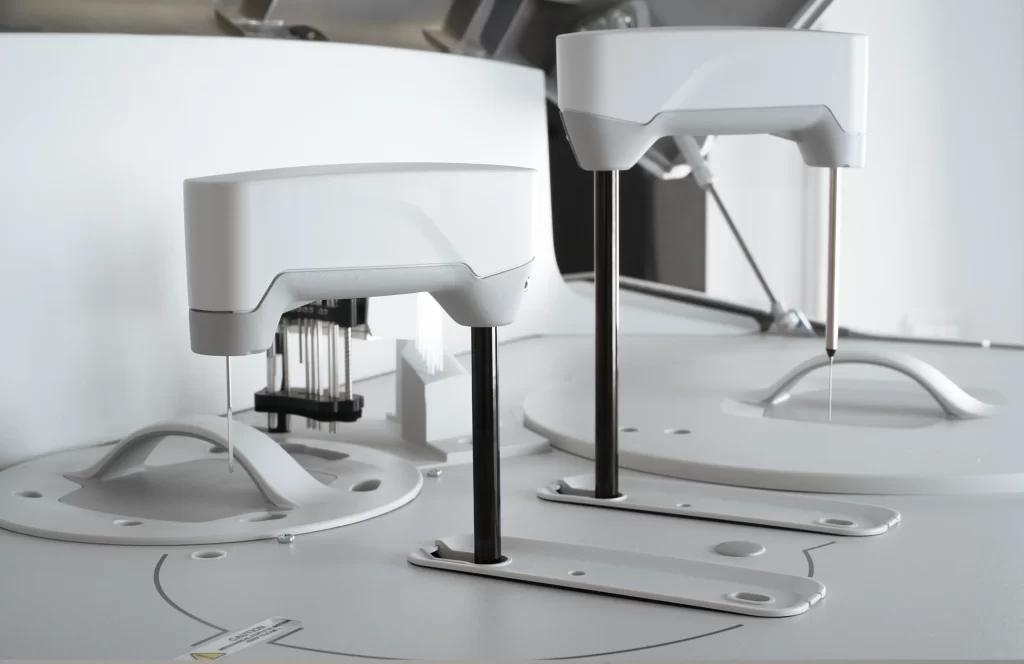
Clinical Lab Systems
Housing parts, tray components, and reagent modules manufactured for dimensional stability and automation integration.
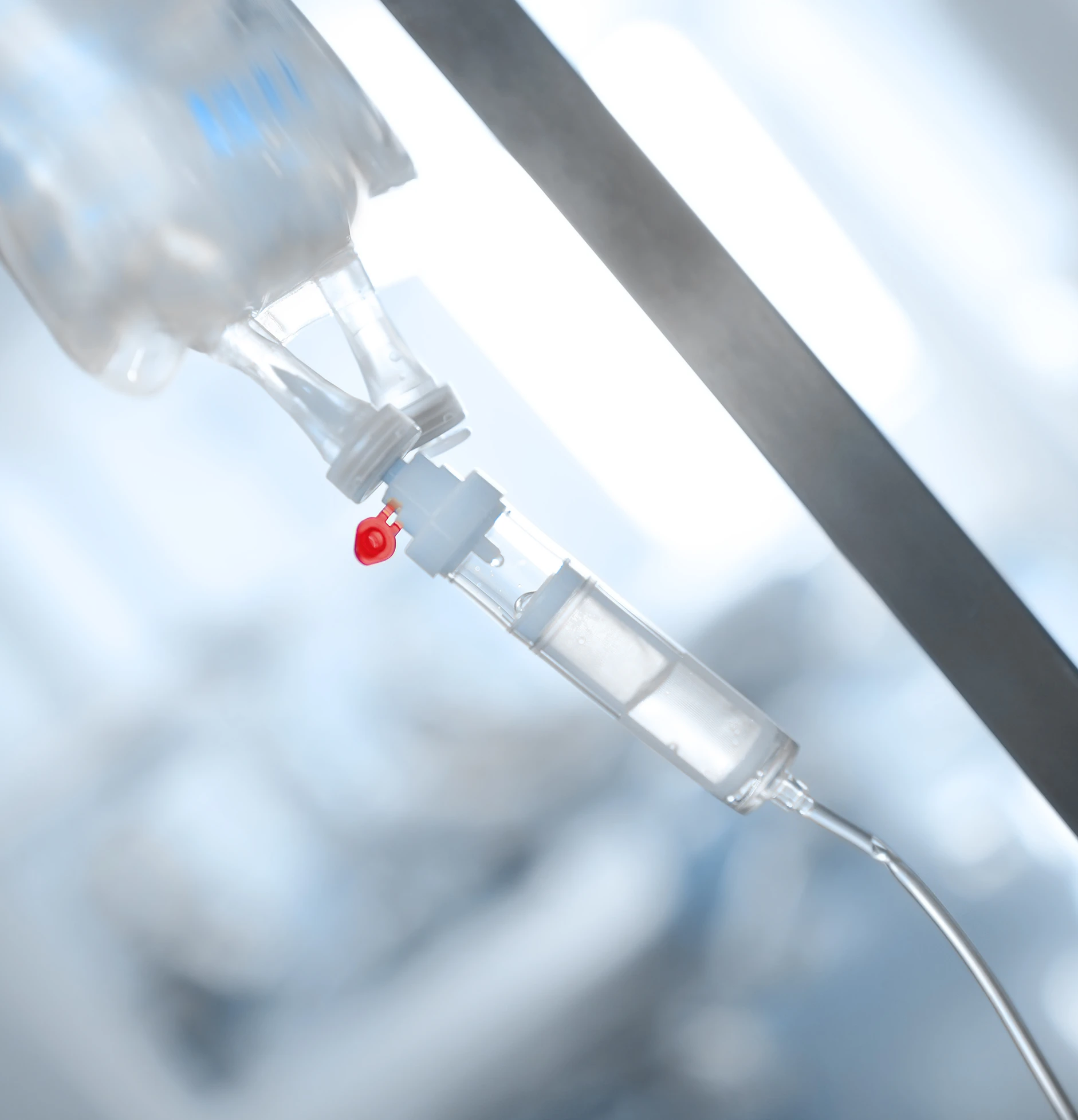
Microfluidic Components
Filters, Channels, wells, seals, and fluidic paths produced with high precision for traceable, biocompatible diagnostic flows.

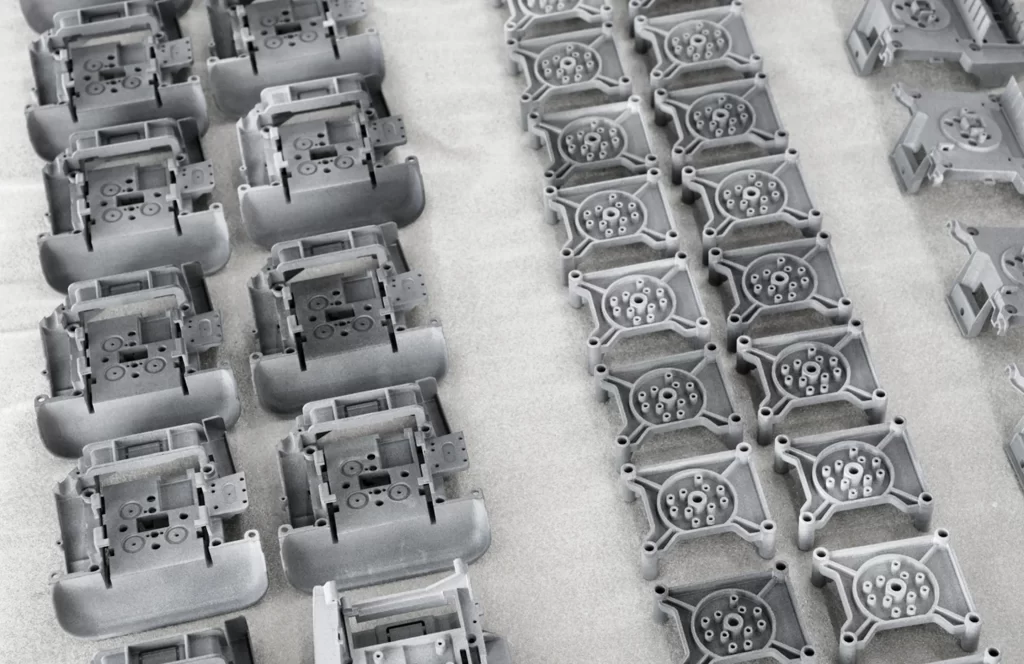
Single-Use Diagnostic
Single-use consumables with ISO7 cleanroom assembly for high repeatability, cost-efficiency, and regulatory compliance in pharma, biotech, and clinical labs.
Integrated Plastic Solutions
Let’s Engineer Your Next Diagnostic Solution
We design, tool and mould critical diagnostic components for OEMs and CDMOs, built to meet global compliance, lifecycle demands and industrial-scale production.
Portfolio & Case Studies
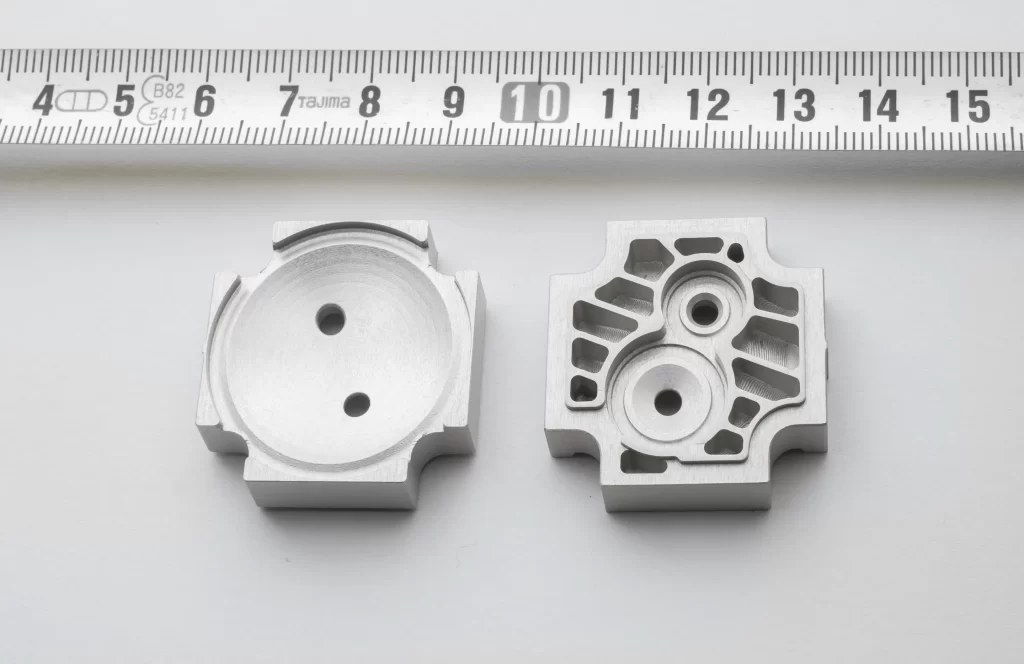
Components BA200 & BA400 - Biochemistry Analysers
Thermoplastic Injection parts & Assembly
Client: Biosystems
Membrane Pump for Biochemistry Analysers
Final parts machining
Client: Biosystems
Automatic Analyzer
Small Serie for tests & validation - 3D printing
Client: Covsense
Precision Manufacturing Partner for Diagnostics Devices
We deliver fully integrated engineering, toolmaking, and ISO 13485-certified injection moulding with cleanroom production, validation expertise, and micro-tolerance tooling built for diagnostic devices.
Tooling Strategy for Low to Mid Cavitation
We design and build moulds optimised for early-stage production and low-volume diagnostics, including 2-to-8 cavity tools, modular platforms, and design-for-repetition strategies that support rapid iteration and clinical rollout.
Micro-Tooling for Dimensional Accuracy
With milling precision down to 1 micron and tooling geometries as small as 0.1mm, our infrastructure supports the tolerance requirements of microfluidic interfaces, fluidic seals, and multi-part alignment in test cartridges and cassettes.
Scientific Moulding with Full Validation Readiness
We incorporate cavity pressure control, thermal optimisation, and decoupled process settings to ensure shot-to-shot repeatability. Moulds are tested in controlled environments using IQ, OQ, PQ validation protocols for regulatory readiness.
Cleanroom-Ready Injection Capacity
Our ISO 7 cleanroom manufacturing supports clean transfer of validated tools into series production, with complete ISIR reports, in-process controls, and traceability aligned to ISO 13485 and customer-specific GMP frameworks.
Design for Manufacturability (DFM) Integration
Our engineering teams work upstream with your device developers to ensure early-stage feasibility, cost-efficient tooling, and accelerated time to industrialisation, reducing correction loops and minimising rework across the lifecycle.